Clinical Trials Support Unit task delegation log. AE, adverse event;... | Download Scientific Diagram

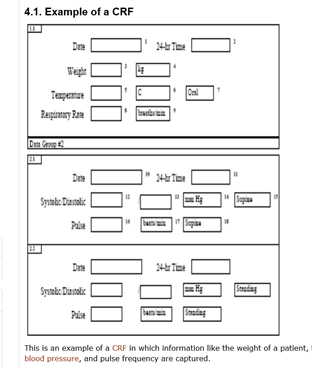
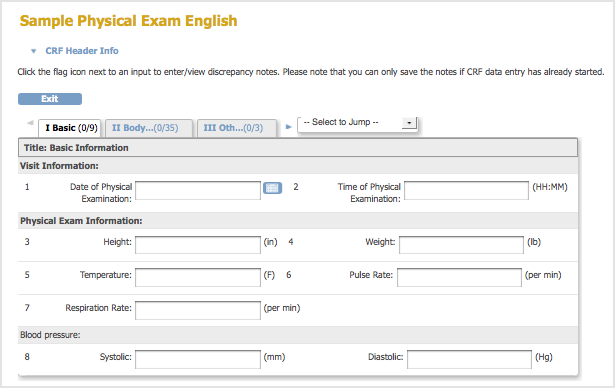
Basics of case report form designing in clinical research | LiMSforum.com – The Global Laboratory, Informatics, Medical and Science Professional Community

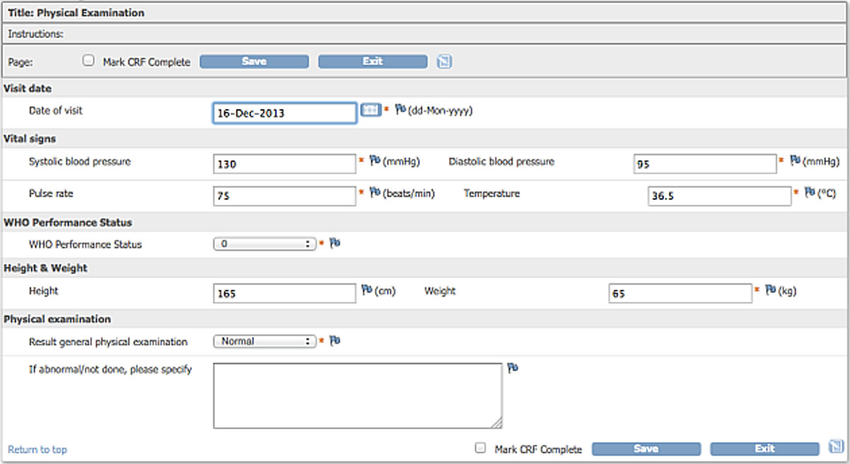
Basics of case report form designing in clinical research Bellary S, Krishnankutty B, Latha M S - Perspect Clin Res

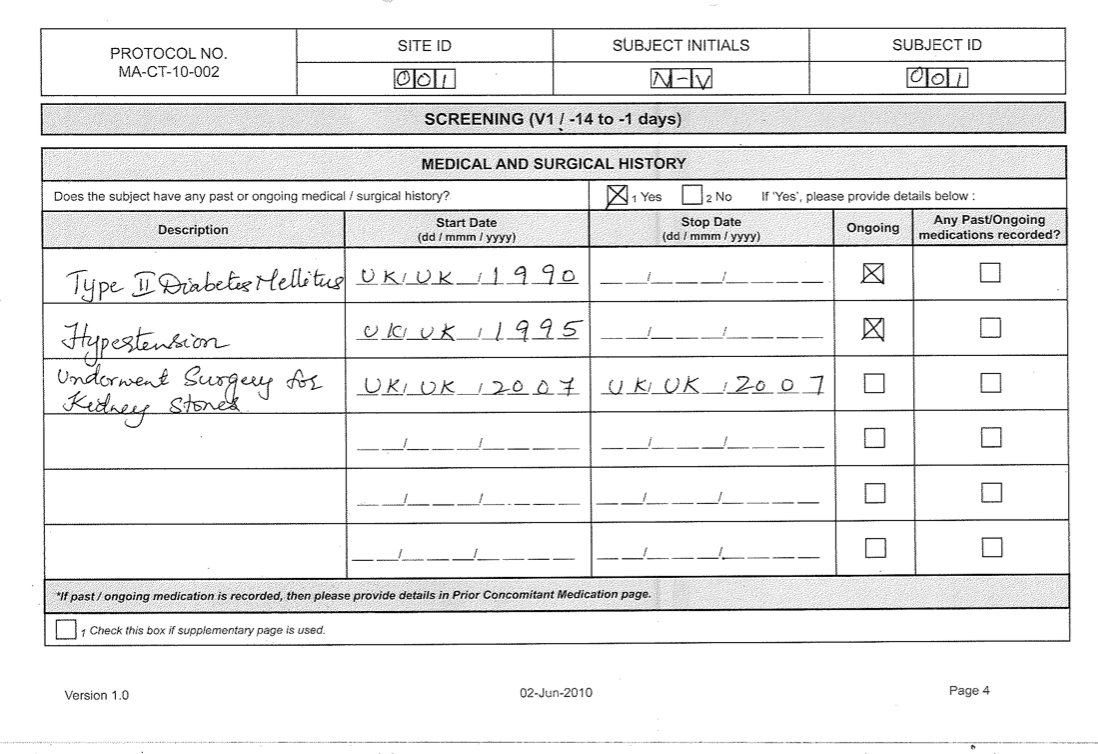
The Case Report Form for the PBRN study "CONDOR Case-Control Study of... | Download Scientific Diagram