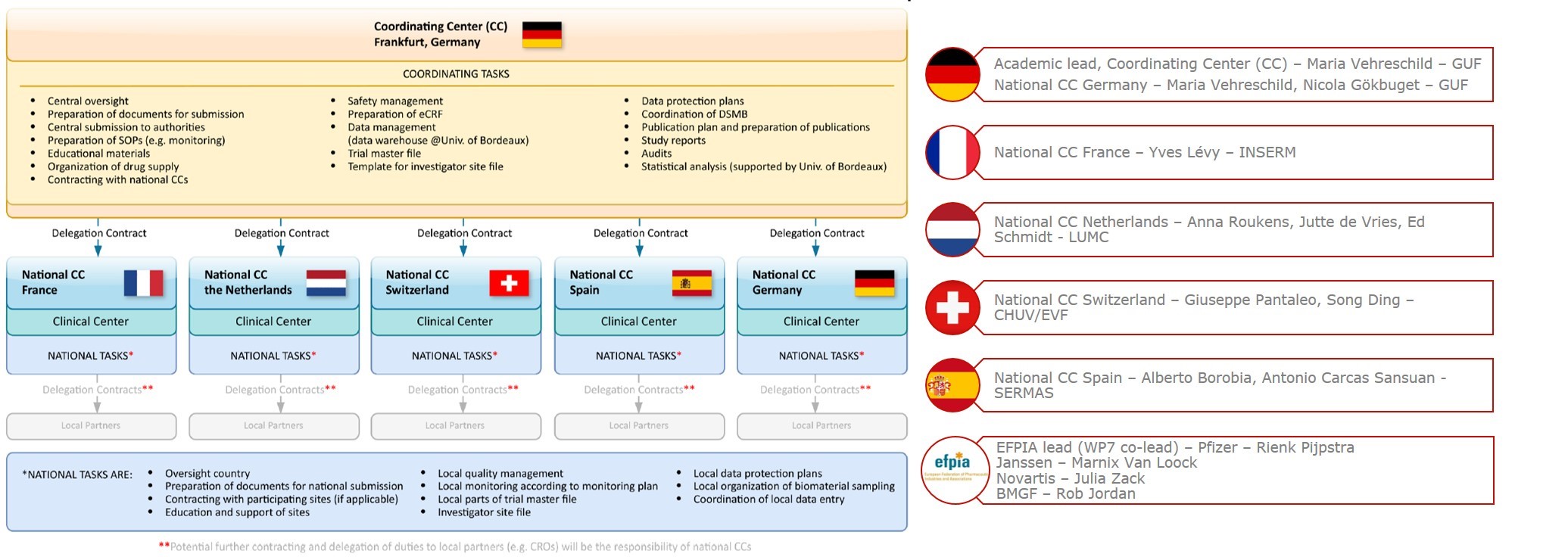
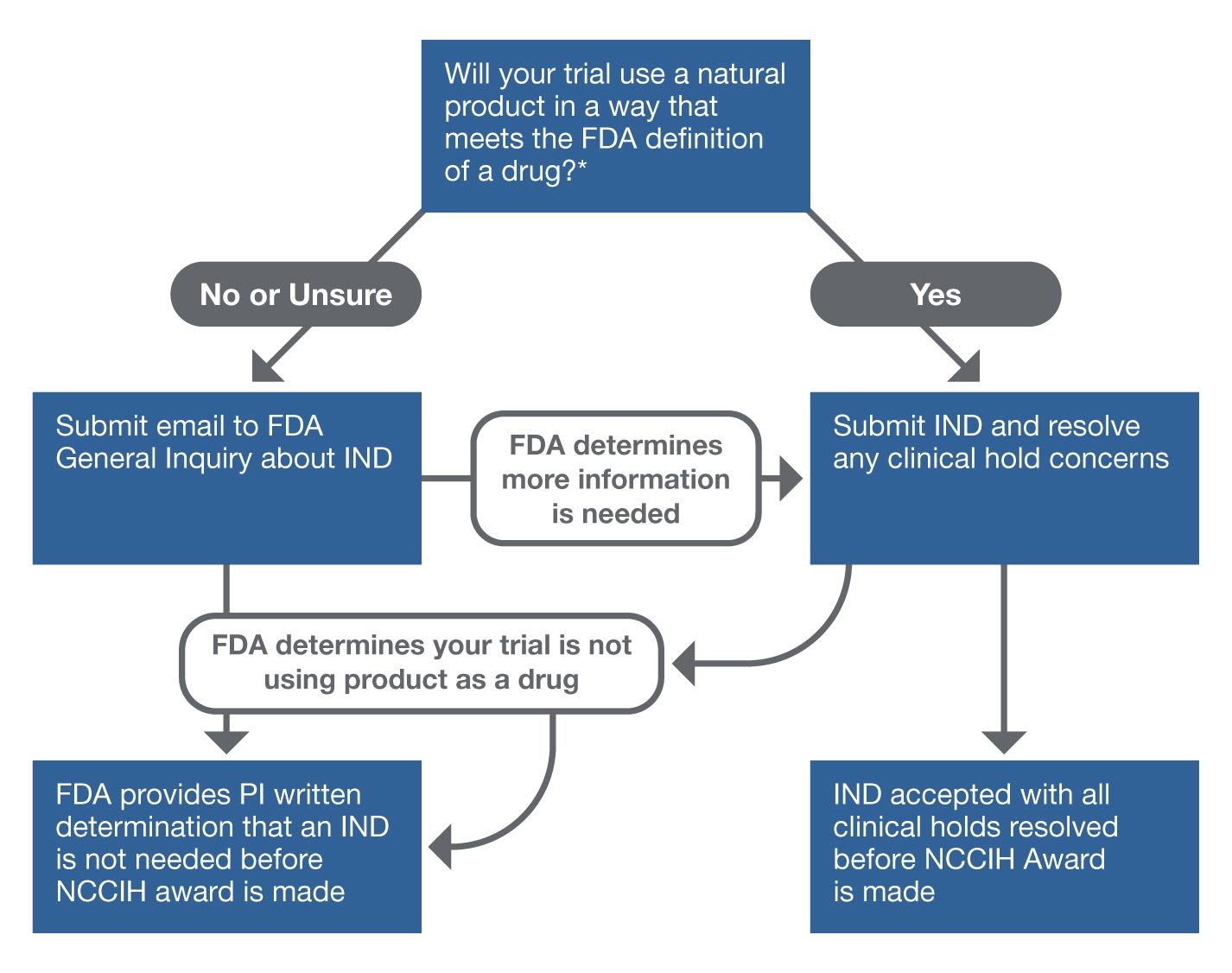
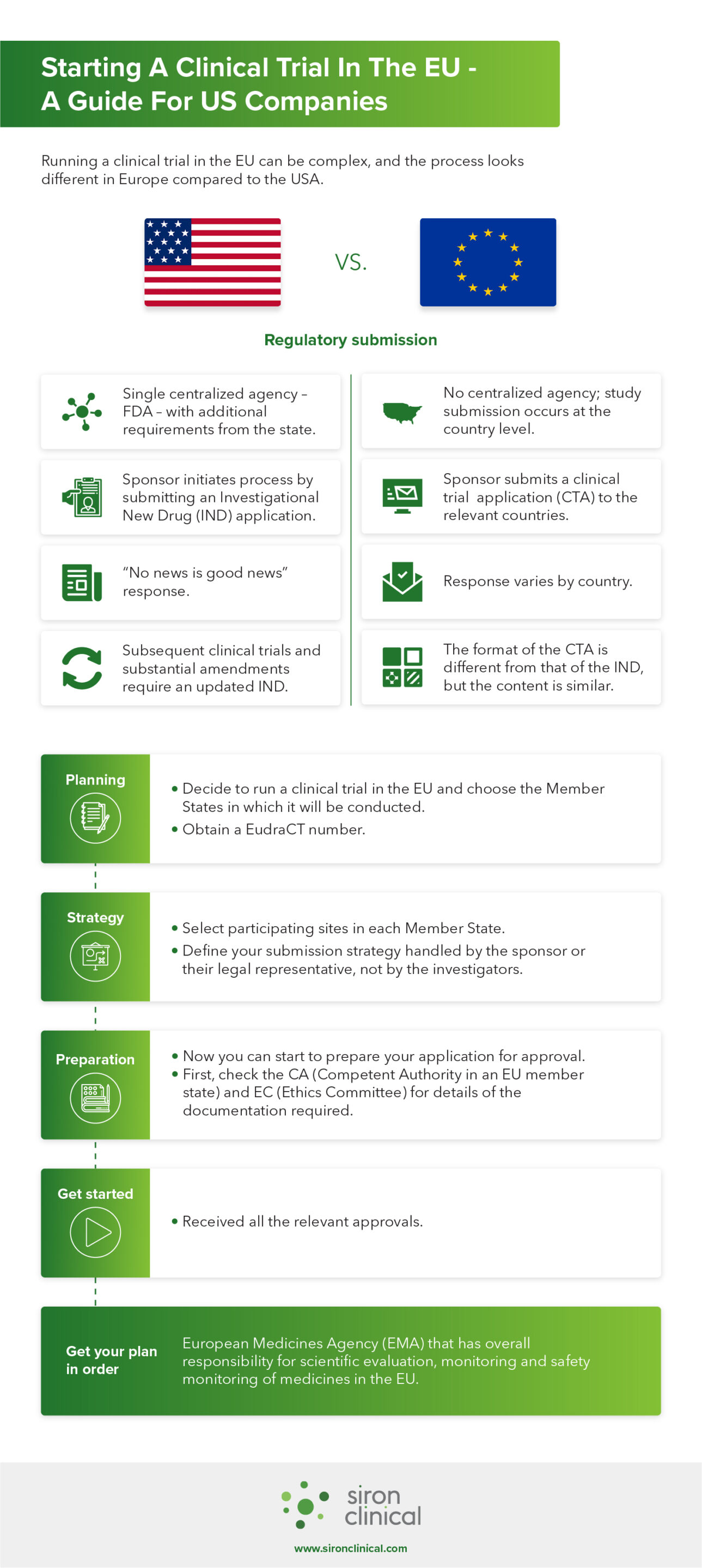
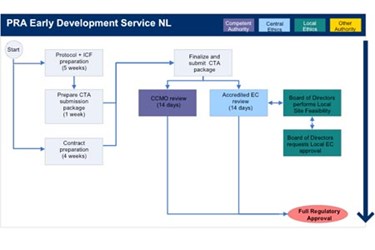
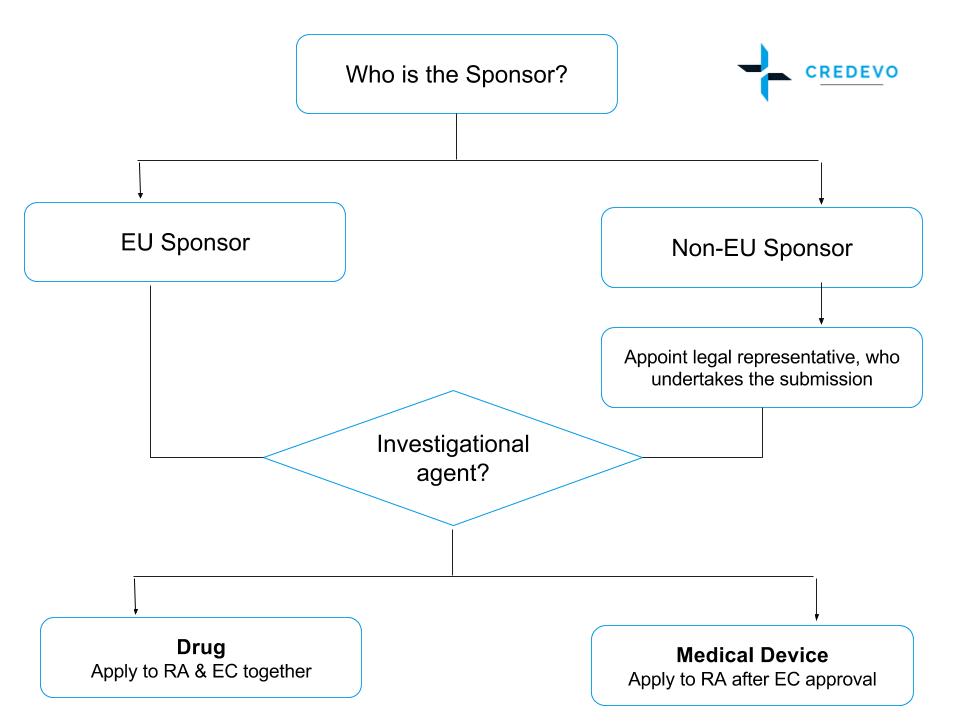
An overview of the procedure for clinical trial applications and the... | Download Scientific Diagram
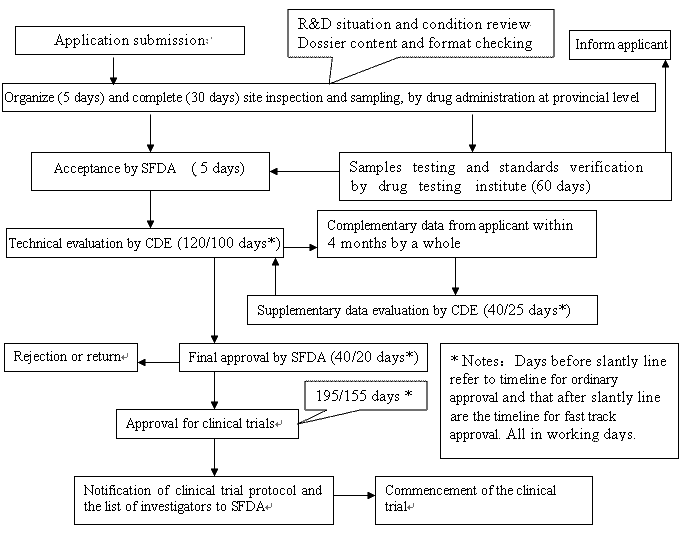
Application and approval procedure for clinical trials-China FDA,SFDA,CFDA,MOH,MOA,AQSIQ,CNCA,CIQ registration approval license for cosmetics,health food supplement,medical device,IVD,drug,infant milk powder,dairy,pet food ,disinfectant etc.

What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH