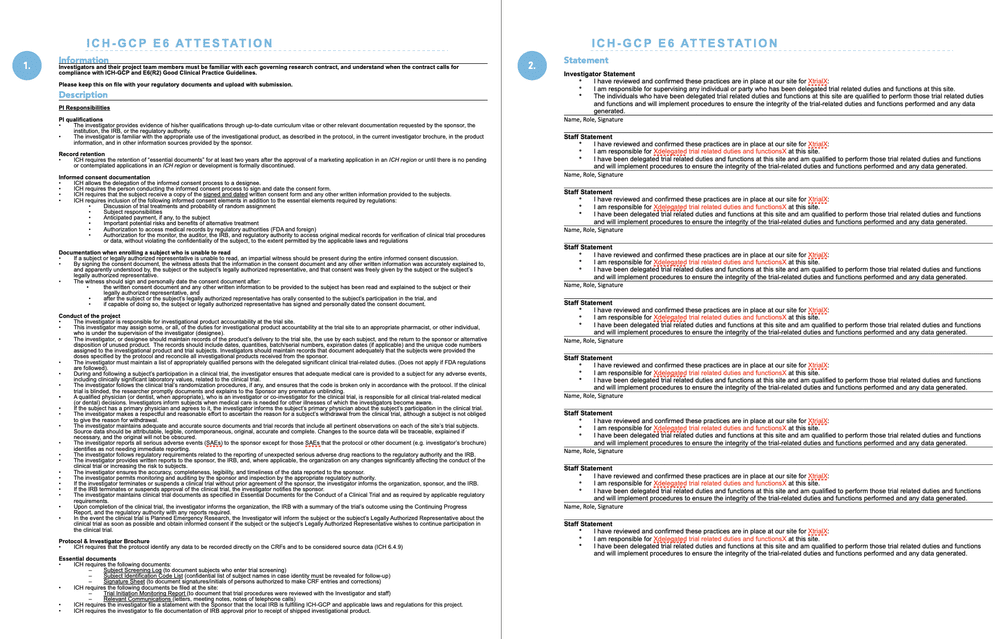
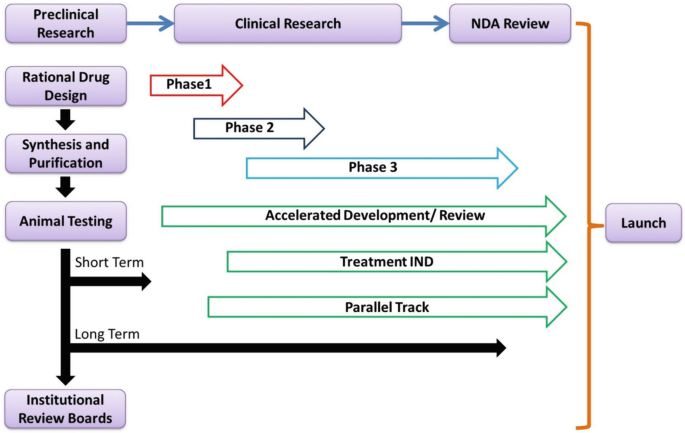
PDF) Central Institutional Ethics Committee needed to facilitate timely review of multicenter clinical trials
ich gcp 3.1.6 — Clinical Research Blog | Certified Clinical Research Professionals Society - Clinical Research Certification
Siemens process control system first product with IEC 62443 security certification | Press | Company | Siemens

Regulatory oversight of cell therapy in China: Government's efforts in patient access and therapeutic innovation - ScienceDirect
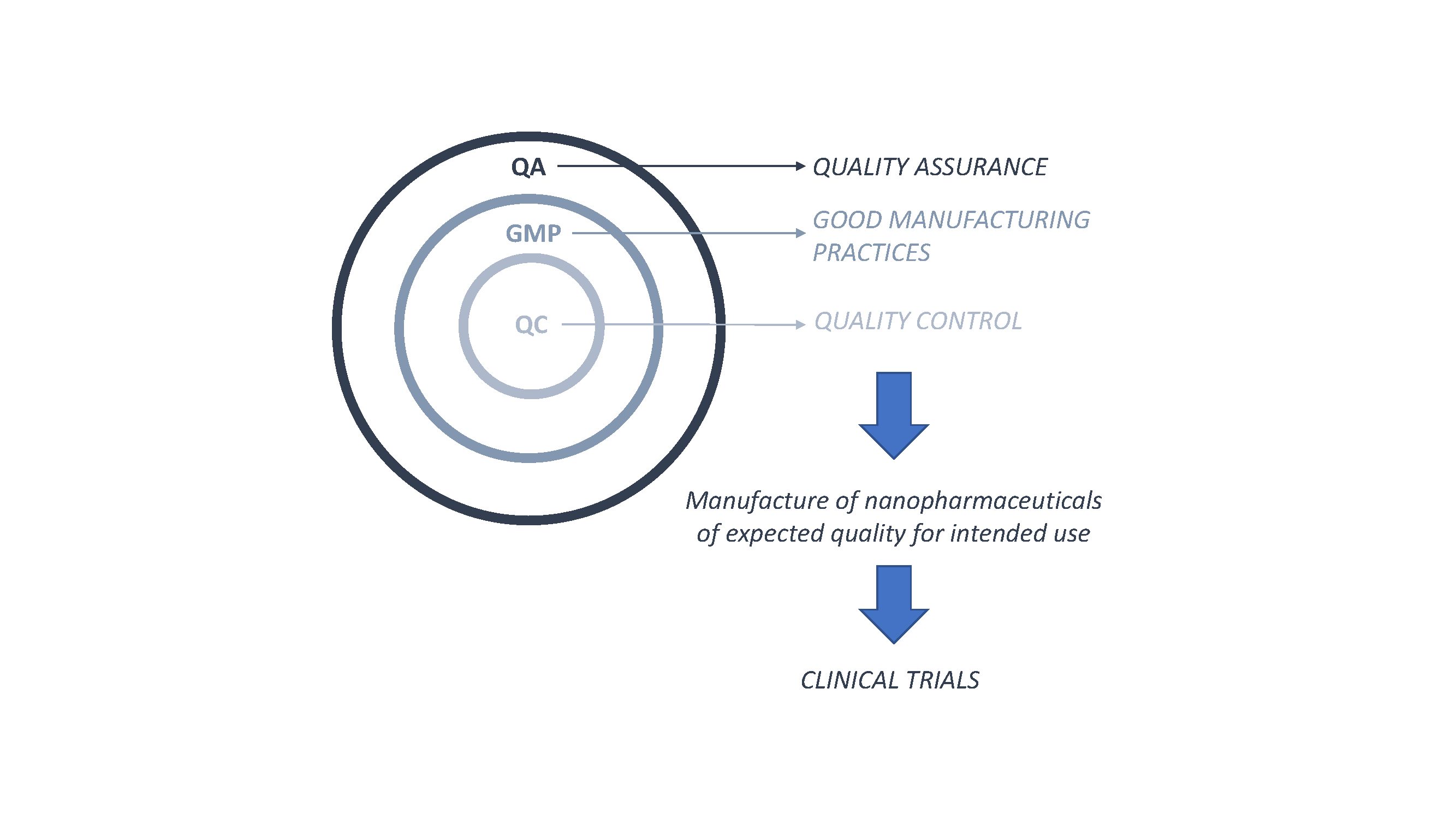
Pharmaceutics | Free Full-Text | Nanopharmaceutics: Part I—Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU | HTML

PDF) Central Institutional Ethics Committee needed to facilitate timely review of multicenter clinical trials